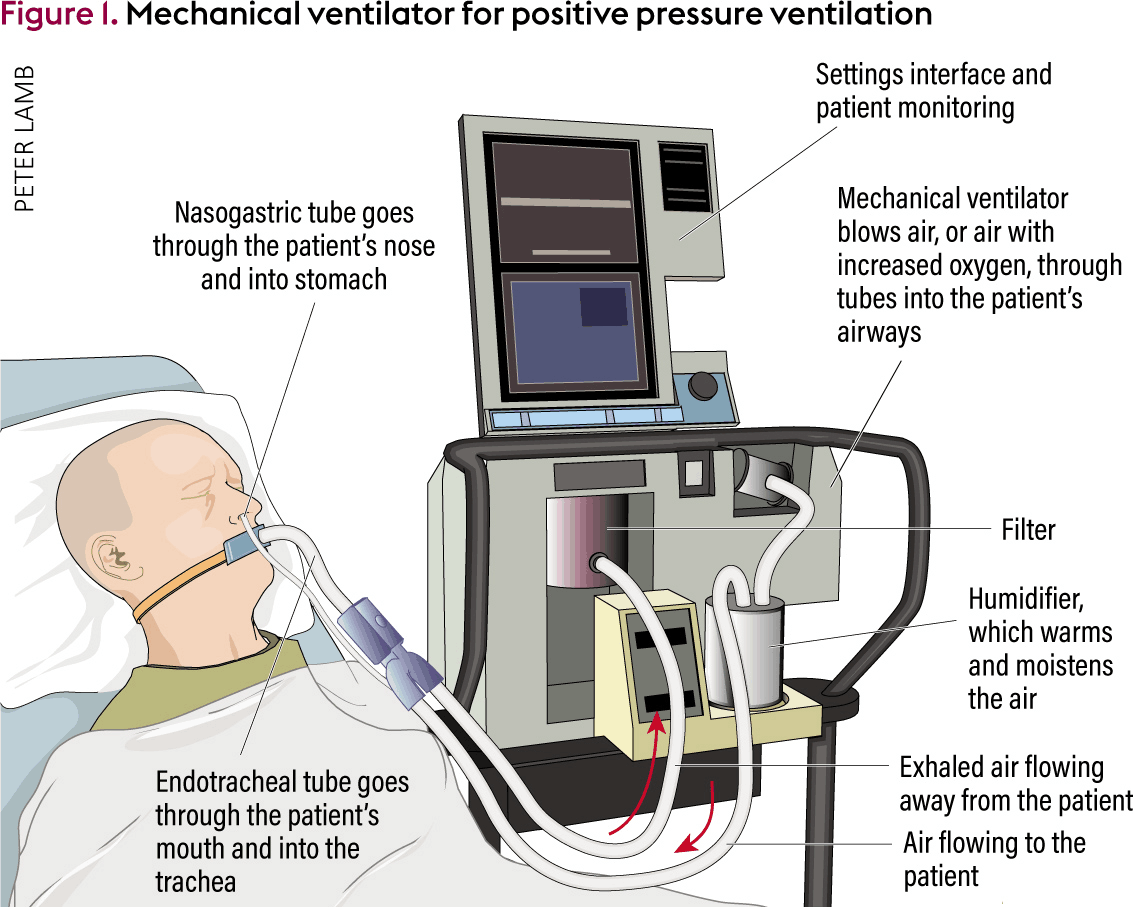
Household non-invasive High-Acuity ventilator expiratory flow diaphragm oxygen gas choke sintered bacterial viral filters
 HENGKO's sintered bacterial viral filters of the ventilator are stainless steel 316, and stainless steel 316L, which have the characteristics of filtering and dustproof. Its material is safe and non-toxic, without odor. The pore diameter is specially designed, and the pore diameter is evenly distributed. It can be used multiple times without cleaning. The air filter material used on the ventilator is strictly selected and tested, and it is suitable for most ventilator models on the market. Large dust particles entering the ventilator may cause wear of the motor bearings of the ventilator, reduce the life of the motor, and increase the noise of the motor.
HENGKO's sintered bacterial viral filters of the ventilator are stainless steel 316, and stainless steel 316L, which have the characteristics of filtering and dustproof. Its material is safe and non-toxic, without odor. The pore diameter is specially designed, and the pore diameter is evenly distributed. It can be used multiple times without cleaning. The air filter material used on the ventilator is strictly selected and tested, and it is suitable for most ventilator models on the market. Large dust particles entering the ventilator may cause wear of the motor bearings of the ventilator, reduce the life of the motor, and increase the noise of the motor.
The ventilator bacterial filter made of ordinary materials may not meet the above performance indicators. Some materials are harmful to the human body and easily cause the human body to react excessively, thereby causing a series of adverse effects on the treatment effect.
All patients are susceptible to the risk of infection after performing pulmonary function tests. Most outpatients visiting pulmonary function departments are not routinely screened for infectious diseases before performing tests. Even when patients are screened, there may be a significant time interval between obtaining culture results and performing the tests. It is tough to identify all the patients with infectious diseases or who are immunocompromised. A recent study showed that 40% of chronic obstructive pulmonary disease (COPD) patients had positive sputum cultures to potentially pathogenic microorganisms. Therefore, stringent universal precautions for everyone needing pulmonary function tests are necessary. Techniques can be used when performing most routine pulmonary function tests. However, the most practical and cost-effective way to ensure that there is no risk of cross-infection between patients is to use bacterial/viral filters.
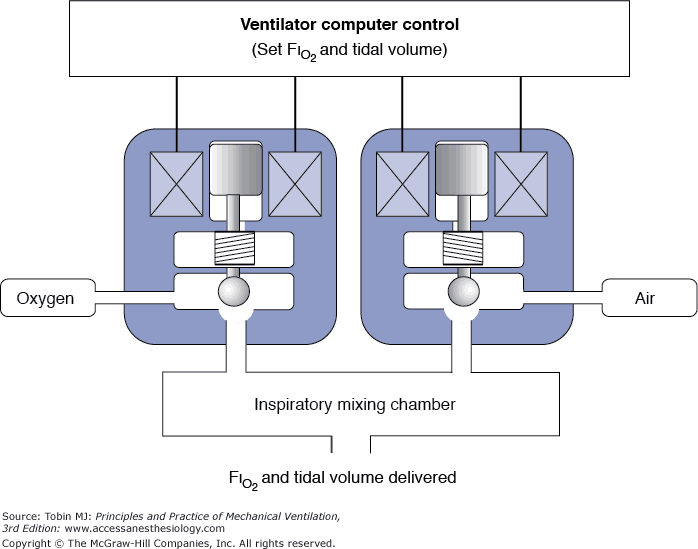
The advantages offered by using bacterial/viral filters are as follows:
Protection of breathing circuits, primarily flow sensors, from contamination with droplets of saliva and mucus that may introduce errors in test measurement, contain microorganisms, and protect patients and staff from inhaling pathogens from breathing circuitry. (Many centers now use staff to perform pulmonary function tests on the equipment (biological controls), which can be used as part of the quality-assurance program.)
A bacterial filter ventilator must have maximal efficiency in trapping and removing bacteria and viruses, but it should also have a low resistance to airflow.
It is generally accepted that respiratory equipment is not sterile and that the risk of exposure to normal levels during testing is no higher than in public places. However, as previously mentioned, some patients have potentially dangerous levels of pathogens in their bodies.
So to minimize the potential risk to patients, it is prudent to use bacterial/viral filters when performing pulmonary function tests. A study by researchers evaluated the effectiveness of single-use bacterial/viral filters in preventing device contamination during pulmonary function assessments. The study's results, which included two groups of patients (infectious and non-infectious), suggest that filters are essential when performing pulmonary function tests because bacteria, including pathogenic bacteria, can spread freely to the device.
The study showed significantly more bacterial growth on the filter's proximal side than on the distal side.
Where and when can bacterial/viral filters be used?
Many pulmonary function departments in hospitals now use bacterial/viral filters, and awareness of the necessity for suitable infection control procedures is well promoted. There are still many hospitals where bacterial/viral filters are not being used, either because of cost or because there is insufficient awareness or knowledge regarding infection control. Nowadays, infection-control nurses are essential in educating staff about the importance of reducing the risk of patients undergoing pulmonary function tests.
Want more information or would like to receive a quote?
Click the Online Service at the top right to contact our salespeople.
E-mail:
ka@hengko.com sales@hengko.com f@hengko.com h@hengko.com